Engineering a Glass-Ceramic Solid Electrolyte Membrane for Reliable and Scalable Electrochemical Lithium Recycling Systems
- Journal
- ACS Applied Energy Materials
- Year
- 2025
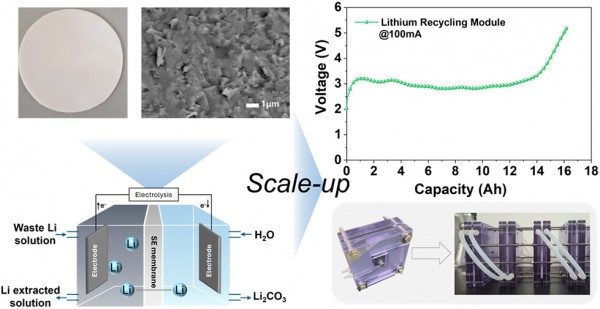
Lithium recycling technology has become increasingly important to address the growing demand for lithium-ion batteries (LIBs) and the limited availability of natural lithium resources. Among various approaches, the electrochemical lithium recycling system has emerged as a promising candidate due to its mild operating conditions and environmental compatibility. In this system, the solid electrolyte (SE) membrane plays a critical role by enabling selective lithium-ion transport while physically separating the electrode compartments. Therefore, SE membranes should possess high ionic conductivity and sufficient density to ensure a stable system operation. However, conventional sol–gel-derived SE membranes often suffer from incomplete densification, undermining the function of the membrane as a physical barrier. In this work, a high-density, high-conductivity lithium aluminum titanium phosphate (LATP)-based glass-ceramic SE membrane is developed via a melt-quenching approach. Optimization of quenching and crystallization conditions yields a SE membrane with a high relative density of 97.1% and an ionic conductivity of 5.06 × 10–4 S cm–1. The optimized SE membrane exhibits a dense microstructure that effectively suppresses liquid leakage and enables a stable electrochemical operation over 100 cycles. Additionally, a scalable bottom-up fabrication strategy based on glass powder processing is established. An integrated prismatic lithium recycling module, constructed by scaling up the SE membrane arrangement from a 1 × 1 to a 3 × 3 configuration and stacking multiple unit cells, yields an approximately 100-fold increase in the available current compared to the single-cell configuration, thereby enhancing the lithium recycling rate per unit time by 2 orders of magnitude.