Redox-Mediated Red-Phosphorous Semi-Liquid Anode Enabling Metal-Free Rechargeable Na-Seawater Batteries with High Energy Density
- Journal
- Adv. Energy Mater.
- Vol
- 11
- Page
- 2102061
- Year
- 2021
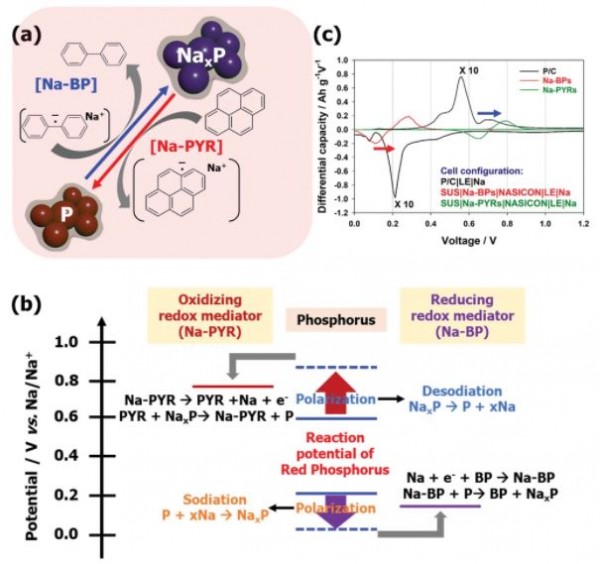
Sodium-seawater batteries (Na-SWB) are considered among the most promising electrochemical devices for large-scale energy storage and the marine sector. In fact, by employing an open-structured cathode, they benefit from the unlimited supply of sodium from seawater. This means, that the energy of such systems is intrinsically limited by the capacity of the anode. In order to increase the energy of Na-SWB, it is therefore necessary to introduce a high-capacity anode such as, e.g., red phosphorus. However, due to its large volume changes upon charge/discharge processes, obtaining thick electrodes and large areal capacity is extremely challenging. Herein, the areal/absolute capacity of the red phosphorus anode is increased by employing a semi-liquid electrode, which includes two redox mediators, i.e., sodium-biphenyl and sodium-pyrene, as reducing and oxidizing species for the exploitation of the full red phosphorus capacity. As a result, the red phosphorus semi-liquid anode in Na-SWB provides a high-capacity of 15 mAh cm–2 in a static anode, showing great energy storage potential for operation in flow-mode when storing the semi-liquid negative electrode in a storage tank.