Using redox electrolytes to extend the charge storage capacity in an aqueous hybrid ion battery
- Journal
- Chemical Engineering Journal
- Vol
- 411
- Page
- 128416
- Year
- 2021
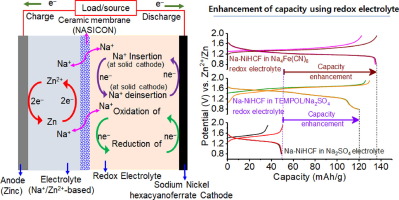
Aqueous hybrid Zn2+/Na+ ion batteries (AHZSIBs) have gained considerable attention for stationary energy storage applications because of their outstanding safety, sustainability, abundance, and low raw material costs. However, the low capacity values (<100 mAh/g) of the Na+ ion deinsertion/insertion cathodes limit the overall capacity storage of AHZSIBs. Herein, we propose a novel concept to extend the charge storage performance of AHZSIBs using electrolyte with redox characteristics. The benefits of using redox aqueous electrolytes such as 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPOL) and sodium ferrocyanide (Na4[Fe(CN)6]) were investigated in an AHZSB, which consists of Zn metal as an anode and sodium nickel hexacyanoferrate (Na-NiHCF) as the Na+ deinsertion/insertion cathode. The proposed AHZSB using Na4[Fe(CN)6] redox electrolyte provided a capacity (144 mAh/g) that was ~2.94 times higher than AHZSIB using a conventional Na2SO4 electrolyte (49 mAh/g). This capacity enhancement emanated from the faradaic contribution of the Fe2+(CN)64−/Fe3+(CN)63− redox pair present in the electrolyte and Fe2+/Fe3+ redox pair in the lattice of Na-NiHCF. In addition, the TEMPOL-based redox electrolyte also improved the capacity (from 49 to 120 mAh/g) through the combined faradaic contribution of the TEMPOL/TEMPOL+ redox pair dissolved in the electrolyte and the Fe2+/Fe3+ redox pair in the Na-NiHCF lattice. These results confirm the competence of the redox electrolyte in AHZSIB in enhancing the charge storage capacity. We anticipate that this proof-of-concept study will provide a new direction for developing high-capacity storage AHZSIBs. More importantly, this approach can be used in any aqueous/non-aqueous batteries.