Analysis of geometric and electrochemical characteristics of lithium cobalt oxide electrode with different packing densities
- Journal
- Journal of Power Sources
- Vol
- 328
- Page
- 46-55
- Year
- 2016
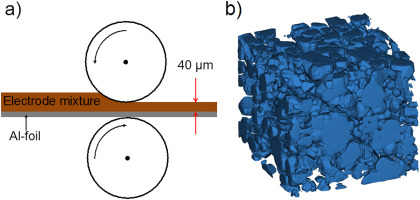
To investigate geometric and electrochemical characteristics of Li ion battery electrode with different packing densities, lithium cobalt oxide (LiCoO2) cathode electrodes were fabricated from a 94:3:3 (wt%) mixture of LiCoO2, polymeric binder, and super-P carbon black and calendered to different densities. A synchrotron X-ray nano-computed tomography system with a spatial resolution of 58.2 nm at the Advanced Photon Source of the Argonne National Laboratory was employed to obtain three dimensional morphology data of the electrodes. The morphology data were quantitatively analyzed to characterize their geometric properties, such as porosity, tortuosity, specific surface area, and pore size distribution. The geometric and electrochemical analysis reveal that high packing density electrodes have smaller average pore size and narrower pore size distribution, which improves the electrical contact between carbon-binder matrix and LiCoO2 particles. The better contact improves the capacity and rate capability by reducing the possibility of electrically isolated LiCoO2 particles and increasing the electrochemically active area. The results show that increase of packing density results in higher tortuosity, but electrochemically active area is more crucial to cell performance than tortuosity at up to 3.6 g/cm3 packing density and 4 C rate.