Na ion- Conducting Ceramic as Solid Electrolyte for Rechargeable Seawater Batteries.
- Journal
- Electrochimica Acta
- Vol
- 191
- Page
- 1-7
- Year
- 2016
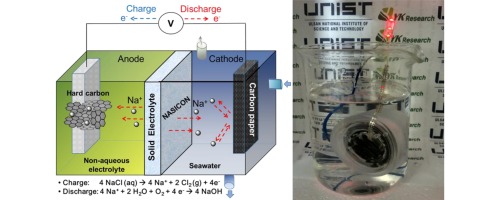
This study describes the assembly of a rechargeable seawater battery using hard carbon as the anode, seawater as the cathode, and a fast Na ion-conducting ceramic as the solid electrolyte. Two different Na ion-conducting ceramics, β″-Al2O3 and Na3Zr2Si2PO12 (NASICON), are used as the solid electrolytes in this study. The discharge capacity of the seawater battery with the NASICON solid electrolyte is 120 mAh g−1 after the first cycle and over 91% coulombic efficiency after twenty cycles. However, under the same experimental conditions, the discharge capacity of the seawater battery with a β"-Al2O3 electrolyte significantly drops to 10 mAh g−1 after one cycle. It is observed that the stability of NASICON in seawater is superior to that of β"-Al2O3 and impedance results of NASICON are not changed significantly compared to that of β"-Al2O3 after cycling tests. The stability of Na ion-conducting ceramics in seawater and their effects on the electrochemical performance of seawater batteries are presented and discussed.