Abstract
A new system of lithium recycling is designed to recover Li from materials containing waste Li. This waste-to-lithium (WTL) system operates based on electrochemical reaction at room temperature using three functional sections: two cathode compartments, one for the waste materials and one for recycling the recovered Li, and one Li-harvesting anode compartment located between the two cathode compartments. By charging the system, Li ions from waste Li-containing materials are extracted and converted into Li metal in the harvesting anode compartment. The harvested Li metal can be transformed via electrochemical reactions with water into useful Li precursors such as LiOH and Li2CO3, which are commonly used Li source chemicals. The concept of the WTL system is proved using well-known cathode materials from Li ion batteries and a commercial Li ion battery pack as the waste Li-containing materials. The harvested Li metal shows a purity of ∼99%, and the produced Li2CO3 is phase-pure without any notable secondary phase. Compared to existing Li recycling technologies, which are generally complicated, time-consuming chemical and heating processes, the WTL system is straightforward and can be operated at room temperature without using any deleterious acid chemicals, thus opening a new avenue for cost-effective, eco-friendly Li-recycling systems.
Export citation and abstract BibTeX RIS
Because the lithium ion battery (LIB) has been widely commercialized as energy storage for portable electronic devices, its production has dramatically increased over the past few decades. In 2009, the sale of LIBs reached close to 10 billion U.S. dollars,1 where ∼70% of LIBs are used in portable electronic devices such as laptop computers and cell phones. When LIB technology is used for electric vehicles (EVs), the battery size increases to approximately a thousand times that of the LIB in a laptop computer. The EV battery generally contains 20–30 kg of lithium (Li).2 Since EV markets are growing exponentially, as shown in Fig. S1 in supplementary material, demand for Li sources is also increasing rapidly. With this trend, the limited amount of the global Li resources (25.5 million tons) is projected to be depleted in 2025.2 In addition, increasing LIB waste will increase environmental costs.3
There is an increasingly urgent need to recycle Li source materials from waste LIBs.4,5 Three types of Li recycling methods are primarily in use at the moment: leaching, extraction, and precipitation.4–13 Leaching recycling methods for Li include acid-leaching and bio-leaching. Acid-leaching which uses strong acids like sulfuric acid, hydrochloric acid, and nitric acid to dissolve waste Li content solids, is the best-known and most widely used Li recycling method.6–8 It has the advantage of having low energy requirements, but is expensive and environmentally unfriendly due to the use of acids. The bio-leaching method uses biodegradable materials to recapture Li source materials from waste LIBs.9,10 This method is economically advantageous, but requires a long treatment period using incubating microbes and also uses acids. The extraction method uses acid extractants such as di-phosphoric materials from waste LIBs through a hydro-metallurgical process.11 This process can be operated with low electrical energy. However, the use of acid extractants is costly and environmentally unfriendly.4 The precipitation method for Li recycling also uses toxic chemical agents to precipitate Li from waste LIBs.4 All these systems share a common crucial problem: the use of toxic acid chemicals for leaching, extraction, and precipitation. These approaches require complicated chemical and heat processes to harvest Li from waste materials, and increase environmental costs due to the acid treatment.12 Nie et al. have recently reported a recycling process of LiCoO2 from spent batteries with a solid state route, which includes multiple steps such as washing, drying, filtering, and heat-treatment.13
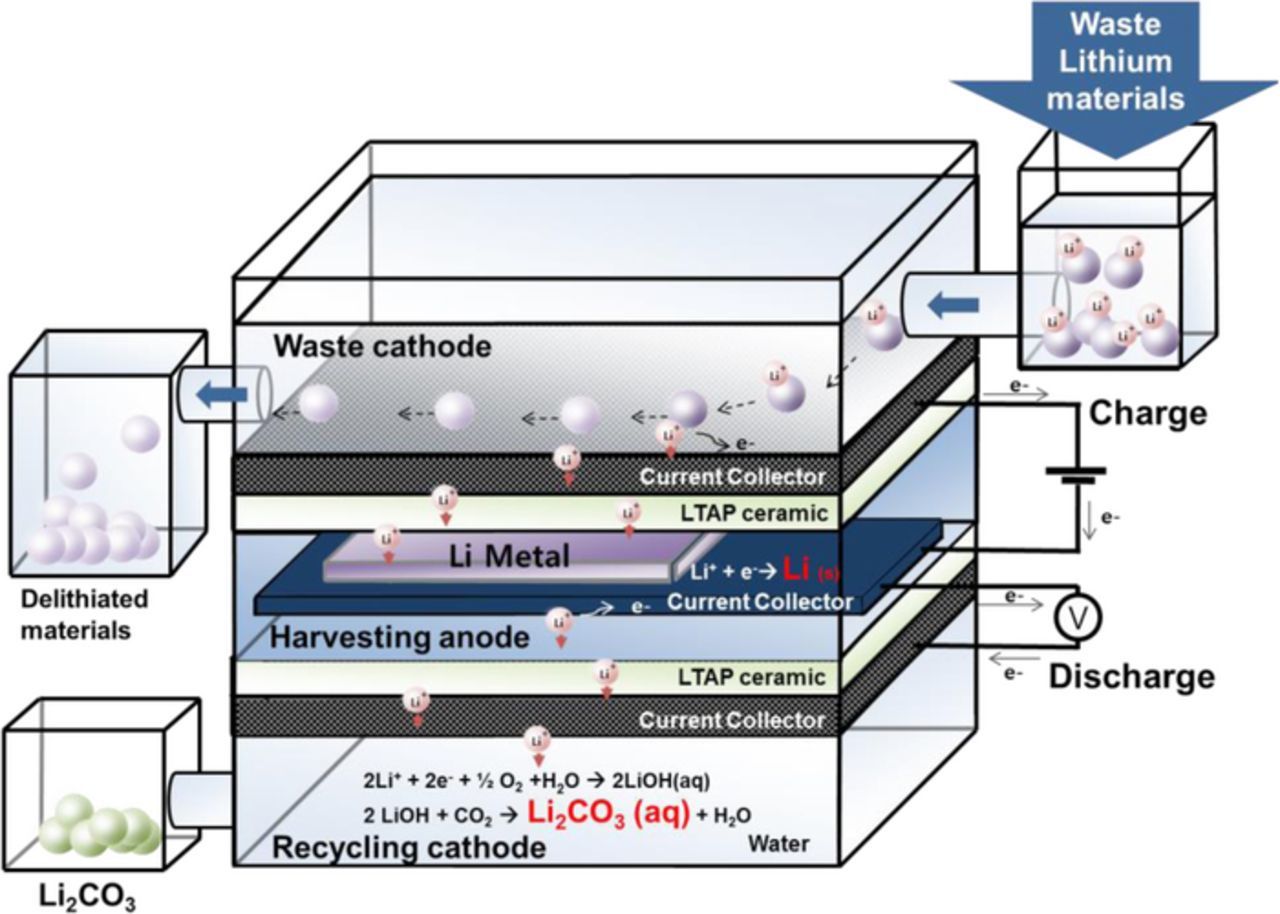
To circumvent these issues with existing Li-recycling technologies, a new type of waste-to-lithium (WTL) recycling system has been proposed in this work (see Fig. 1). This WTL system can easily harvest Li metal from waste Li-containing materials by an electrochemical route using a charge process. Furthermore, through the discharging process, the harvested Li metal can be recycled into commercially applicable air-stable Li precursors, such as LiOH and Li2CO3, via its reactions with H2O.14 Our approach is straightforward, and the system operates at room temperature without using any deleterious acid solutions, which suggests that this WTL system is a promising way to construct an economical and environmentally friendly Li recycling system.
Figure 1. A schematic diagram of the WTL system. Li ions of the waste Li materials in the waste cathode compartment move to the harvesting anode compartment and form Li metal when the system is charged. During discharging of the system, Li2CO3 or LiOH can be produced in the recycling cathode compartment by reacting with H2O, O2, and CO2.
Experimental
Preparation of WTL cell components
1 M LiPF6 in ethylene carbonate–dimethyl carbonate (EC-DMC) (1 : 1 volume %) as an organic liquid electrolyte was purchased from Panaxetec Corp. Li ion conducting glass ceramic plate, Lithium aluminum titanium phosphate (LATP) solid membranes with 19 mm diameter and 800 μm thickness were purchased from OHARA Inc. The ionic conductivity of the LATP was measured to be ∼10−4 S·cm−1 at room temperature. A polyethylene separator with a thickness of 25 μm was purchased from Celgard. LiFePO4, LiMn2O4 and LiNi1/3Co1/3Mn1/3O2 powders (MTI Corp.) were used as Li-containing materials for Li harvesting tests. A nickel mesh (Alfa Aesar) and graphite (Sigma-Aldrich) were used for the WTL anode compartment as the current collector and the Li-harvesting electrode, respectively. The graphite powder was uniformly mixed with a carbon black conducting agent (TIMCAL Super P) and a polyvinylidenedifluoride binder at a weight ratio of 80:10:10 in N-Methyl-2-pyrrolidone, and then cast on Cu foil using a doctor blading method, followed by drying in an oven at 100°C and pressing into thin sheets. Carbon papers (JNTG Corp.) with a thickness of 370 μm and an electrical resistivity less than 20 Ω-mm were used as the current collector in the cathode compartment (see Fig. 1).
Fabrication of the WTL cell
The WTL system consists of three parts: the waste cathode compartment (top), the harvesting anode compartment (middle), and the recycling cathode compartment (bottom), as schematically illustrated in Fig. 1. In order to completely separate the three compartments, a dense Li ion conducting ceramic membrane (LATP) was applied. First, the harvesting anode compartment was assembled in an argon-filled glove box (under 3 ppm of H2O and O2) using a Ni mesh as the current collector and 1 M LiPF6 in EC–DMC as a non-aqueous electrolyte. The Ni mesh and LATP were completely separated with a polymer separator (polyethylene) to prevent chemical reaction between the LATP and the harvested Li metal. Both sides of the anode compartment were enclosed with two different LATPs, whose exposed surfaces with a diameter of 16 mm face each cathode compartment. The assembled anode compartment was moved out of the glove box and inserted into the middle of a specially designed plastic three-chamber container: the top waste cathode, the bottom recycling cathode, and the middle space between them for the harvesting anode. Carbon paper current collectors were inserted and fixed onto each LTAP surface of the anode compartment. The waste cathode compartment was filled with distilled water, and 1 M LiPF6 in EC–DMC (0.4 ml) was added as an additive to help the Li ion conduction. On the other hand, the recycling cathode compartment was filled with only distilled water. The two cathode compartments have open aerated system.
Testing of WTL cell
Well-known cathode materials, such as LiFePO4, LiMn2O4, and LiNi1/3Co1/3Mn1/3O2 powders were put into the waste cathode compartment. Then, the cathode materials sunk down through the water onto the carbon paper, resulting in physical contact between them. The WTL cell was tested with a constant current of 0.05 mA. An electrical charge process was performed to extract lithium from the cathode materials. As soon as the charge process ended, the harvesting anode compartment was handled in two ways. First, it was moved into the glove box and disassembled to collect lithium metal for analysis. Second, the harvesting anode compartment was electrically connected with the recycling cathode compartment, and a discharge process was carried out to form LiOH (aq) or Li2CO3 (aq). The solution processed in the recycling cathode compartment was then dried to acquire the powders in a vacuum oven at 80°C for 1 day. As a practical example of waste Li-ion battery, a used smart phone battery pack (3.8 V, TES-Global Corp.) was employed to examine the Li-harvesting.
Characterizations
The X-ray diffraction (XRD) powder pattern was obtained with an X-ray diffractometer (D/Max2000, Rigaku). Rietveld refinement was carried out in the 2θ range = 10−80°, with a 0.5° step interval and a 10 s step time. The surface morphologies were observed by S-4200 Hitachi field-emission SEM. The ionic conductivities were measured by an OAKTON con 11 meter kit. The ICP/MS measurement was performed by 700-ES from Varian Inc. The electrochemical tests were performed by an automatic Galvanostatic charge-discharge unit (WonATech. Co.)
Results and Discussion
Fig. 1 shows the schematic configuration of the WTL cell used in this study. The key strategy in this cell is the use of a multi-layer electrolyte system,15,16 which is achieved through the use of three compartments in the cell: the waste cathode compartment, the harvesting anode compartment, and the recycling cathode compartment. The multi-layer electrolyte consists of an aqueous liquid electrolyte in the waste cathode compartment, a non-aqueous liquid electrolyte in the harvesting anode compartment, a water catholyte in the recycling cathode compartment, and a dense ceramic electrolyte between each compartment, as shown in Fig. 1. The use of a ceramic electrolyte prevents direct contact between the liquids. In this work, commercially available Li1+x+yTi2−xAlxP3−ySiyO12 (LTAP) was used as the ceramic electrolyte, with 1 inch × 1 inch-area and 800 μm-thickness. Waste materials containing Li ions, such as the cathodes made of LixFePO4 or LixCoO2, graphite anodes (LixC6), or organic liquid electrolytes (1 M LiPF6 in EC-DEC) can be stored in the waste cathode compartment of the WTL cell. Li-containing inorganic solids will sink due to their higher density (>3.5 g/cm3) than water (1.0 g/cm3) and make physical contact with the current collector located at the bottom of the waste cathode compartment. By charging the WTL cell, the Li metal is harvested by extraction from one of the waste Li-containing materials. After Li-harvesting process, the Li metal can be transformed to Li precursor chemicals such as LiOH and Li2CO3 by electrochemical reaction with H2O in the recycling cathode compartment by discharging the WTL cell.
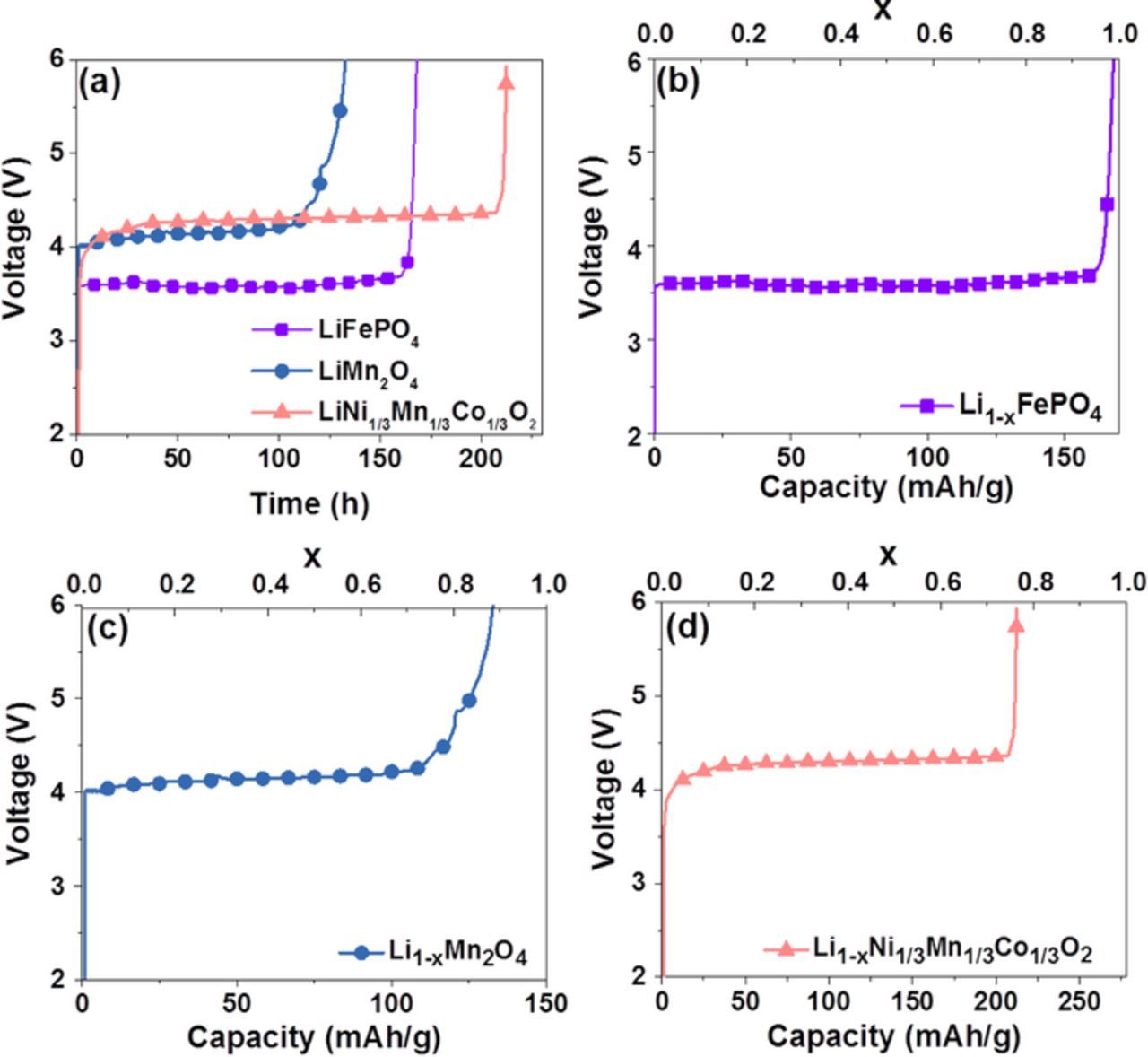
To prove the concept of the WTL system, commercialized solid cathodes such as LiFePO4, LiMn2O4, and LiNi1/3Co1/3Mn1/3O2 were chosen as waste Li-containing materials. 0.05 g of each sample powder was loaded in the waste cathode compartment, and the cell was charged a current of 0.05 mA/cm2. Fig. 2a shows the charge voltage curves for LiFePO4, LiMn2O4, and LiNi1/3Co1/3Mn1/3O2 powders. The charge voltage curve corresponding to Li extraction is observed to be at 3.5 V for LiFePO4, 4.0 V for LiMn2O4, and 4.0 V for LiNi1/3Co1/3Mn1/3O2. The specific charge capacity is measured to be 157 mAh/g for LiFePO4, 111 mAh/g for LiMn2O4, and 210 mAh/g for LiNi1/3Co1/3Mn1/3O2. It is noted that theoretical specific capacity is 170, 148, and 278 mAh/g for LiFePO4, LiMn2O4, and LiNi1/3Co1/3Mn1/3O2, respectively. This means that 92% (see Fig. 2b), 75% (see Fig. 2c), and 76% (see Fig. 2d) of Li ions, relative to the theoretical capacity, are extracted from LiFePO4, LiMn2O4, and LiNi1/3Co1/3Mn1/3O2, respectively, during the charge of the WTL cell.
Figure 2. (a) Charge voltage curves of the WTL cell containing of LiFePO4, LiMn2O4, and LiNi1/3Mn1/3Co1/3O2. Charge capacity and its corresponding Li extraction ratio for (b) Li1−xFePO4, (c) Li1−xMn2O4, and (d) Li1−xNi1/3Mn1/3Co1/3O2.
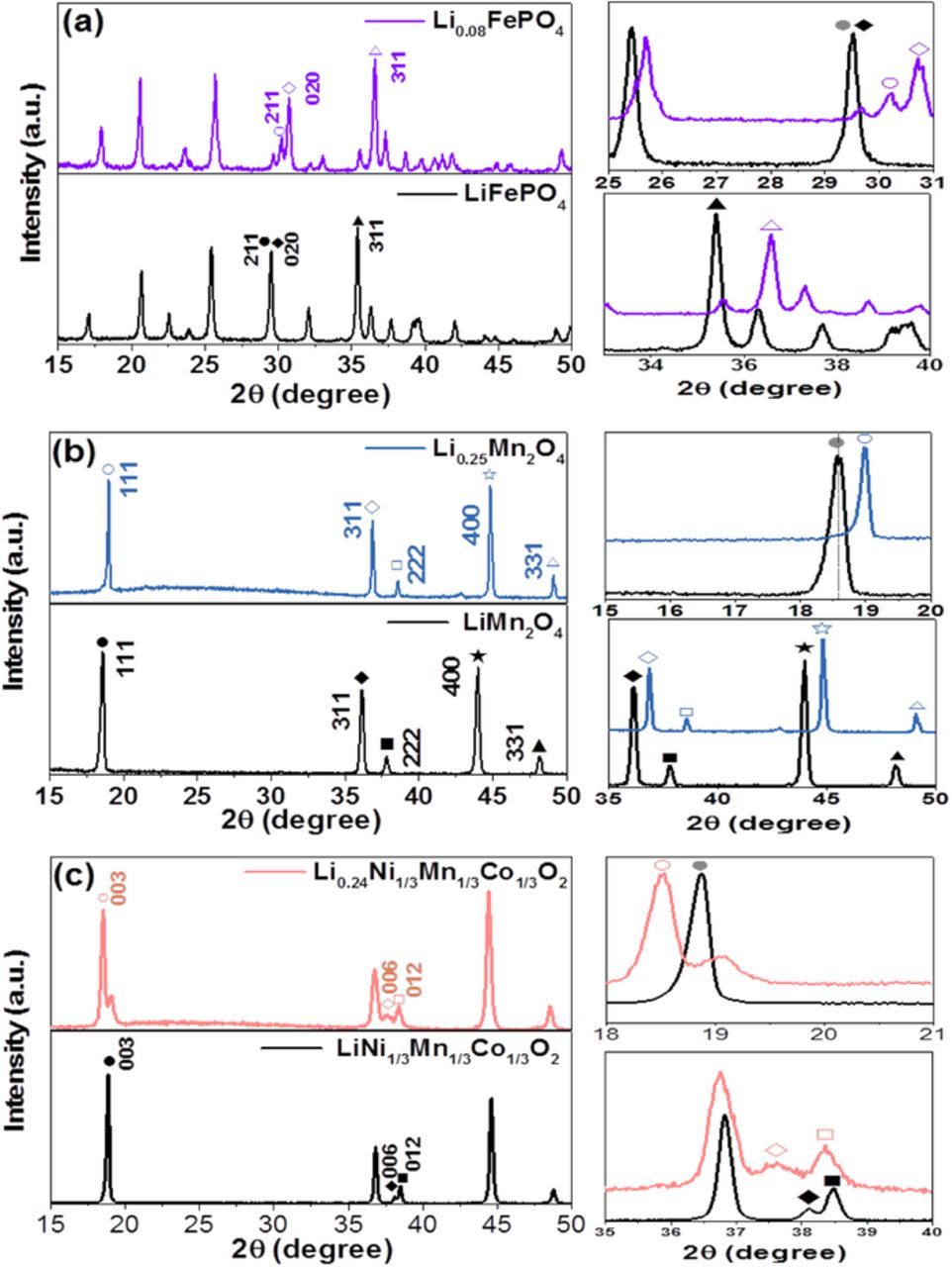
To confirm the electrochemical delithiation from Li solids during the charging process, each sample power was collected after charging the cell and its powder X-ray diffraction (XRD) pattern was measured and compared with its initial XRD pattern as shown in Fig. 3. The change in the crystal structure was investigated. For the LiFePO4 sample, our XRD analysis showed orthorhombic olivine structure with lattice parameters of a = 10.30 Å, b = 5.98 Å, and c = 4.68 Å, which are in agreement with literature.17,18 After charging the cell, the XRD pattern for the Li1−xFePO4 sample is changed, as shown in Fig. 3a; the (211) and (020) peaks of LiFePO4 sample at ∼30° are slightly shifted to greater than 30°, and the (311) peak is also slightly shifted from 35.6° to 36.5° after delithiation of LiFePO4. The XRD pattern of the delithiated Li0.08FePO4 sample with the refined lattice parameters (a = 9.87 Å, b = 5.83 Å, and c = 4.82 Å) is consistent with that of FePO4 phase reported in literature.17,18 After charging of the WTL cell, the Li extraction from the Li1−xMn2O4 was also proved by XRD measurement as shown in Fig. 3b. The initial spinel structure of the LiMn2O4 with the space group Fd3m remains, but its lattice parameter of 8.216 Å is slightly changed to 8.02 Å for the delithiated Li0.25Mn2O4. This is consistent with the XRD pattern of the delithiated Li1−xMn2O4 reported in literature.19,20 The XRD patterns of the LiNi1/3Co1/3Mn1/3O2 and delithiated Li0.25Ni1/3Co1/3Mn1/3O2 are compared in Fig. 3c. Its initial XRD pattern is indexed to the R m space group with lattice parameters of a = 2.84 Å and c = 14.14 Å, which are in agreement with those in literature.21 After delithiation of Li1−xNi1/3Co1/3Mn1/3O2, the c lattice parameter increases to 14.38 Å, which indicates the electrostatic repulsion among the MO2 (M = Ni, Co, Mn) slabs along the z-axis-direction of the unit cell after Li extraction.21 The XRD patterns of the delithiated Li0.25Ni1/3Co1/3Mn1/3O2 are also in accord with that reported in literature.21 The XRD results corroborate the electrochemical Li-extraction behaviors of the Li solids during charging of the WTL cell (Fig. 2).
m space group with lattice parameters of a = 2.84 Å and c = 14.14 Å, which are in agreement with those in literature.21 After delithiation of Li1−xNi1/3Co1/3Mn1/3O2, the c lattice parameter increases to 14.38 Å, which indicates the electrostatic repulsion among the MO2 (M = Ni, Co, Mn) slabs along the z-axis-direction of the unit cell after Li extraction.21 The XRD patterns of the delithiated Li0.25Ni1/3Co1/3Mn1/3O2 are also in accord with that reported in literature.21 The XRD results corroborate the electrochemical Li-extraction behaviors of the Li solids during charging of the WTL cell (Fig. 2).
Figure 3. XRD patterns for initial and delithiated samples of (a) LiFePO4, (b) LiMn2O4, and (c) LiNi1/3Mn1/3Co1/3O2. The right panels show enlarged views of the characteristic peaks of the corresponding samples.
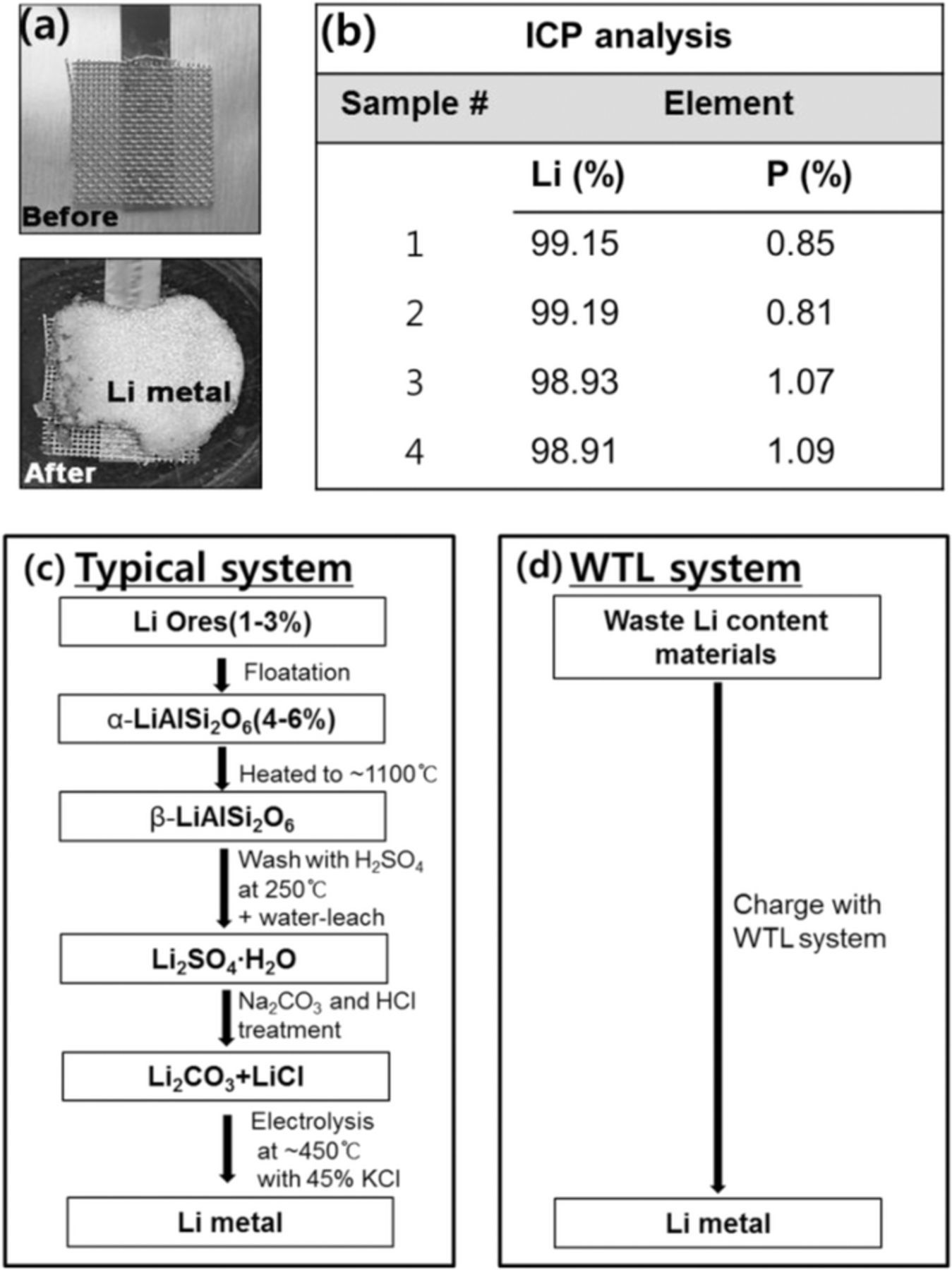
After charging of the WTL cell, Li metal with a shiny surface and gray color was observed on the current collector when the cell was disassembled in the glove box (Fig. 4a). The purity of the harvested Li metal was characterized by ICP. Fig. 4b shows that its purity is 98.9–99.2% Li with 0.81–1.09% phosphorus. The phosphorus might be introduced from the liquid electrolyte of LiPF6 in EC-DMC. In this work, we added a small amount of LiPF6 in the waste cathode compartment by simulating practical conditions in which ordinary Li-ion cells contain liquid electrolytes, such as LiPF6 in EC/DMC, in addition to anode and cathode materials. However, LiPF6 can quickly react with water and form toxic HF (LiPF6 + H2O → POF2(OH) + LiF + 3HF), which may induce safety issues in the system. This issue can be resolved by using neutralizing agents, such as KOH and CaCO3, for HF.
Figure 4. (a) Li metal formed on the Ni current collector after charging WTL. (b) ICP analysis data of harvested Li metal from WTL cell. Flow charts of (c) a typical Li-metal production system22 and (d) WTL system.
The Li metal obtained from the WTL cell has a purity of ∼99%, similar to commercially available Li metal (Aldrich, lithium granular 99%). However, commercial Li metal is generally produced from Li ores after undergoing complicated chemical and heat-treatment processes.22 Fig. 4c shows a typical method for Li metal production. First, Li ores containing 1–3% Li are converted to α-LiAlSi2O6 by the flotation method to increase its Li concentration, and α-LiAlSi2O6 is heated at ∼1100°C to form β-LiAlSi2O6, which has a lower density than the α-phase. Then, β-LiAlSi2O6 is washed with H2SO4 and water-leached at 250°C to form Li2SO4·H2O, followed by treatment with Na2CO3 and HCl to produce Li2CO3 and LiCl. Finally, by electrolysis of the mixture at ∼450°C in an argon atmosphere, commercial Li metal is obtained. Although the two approaches for production of pure Li metal utilize different raw materials, the WTL system (Fig. 4d) can produce high-purity (∼99%) Li metal via a straightforward, one-step electrochemical process at room temperature, compared to the typical process (Fig. 4c).22
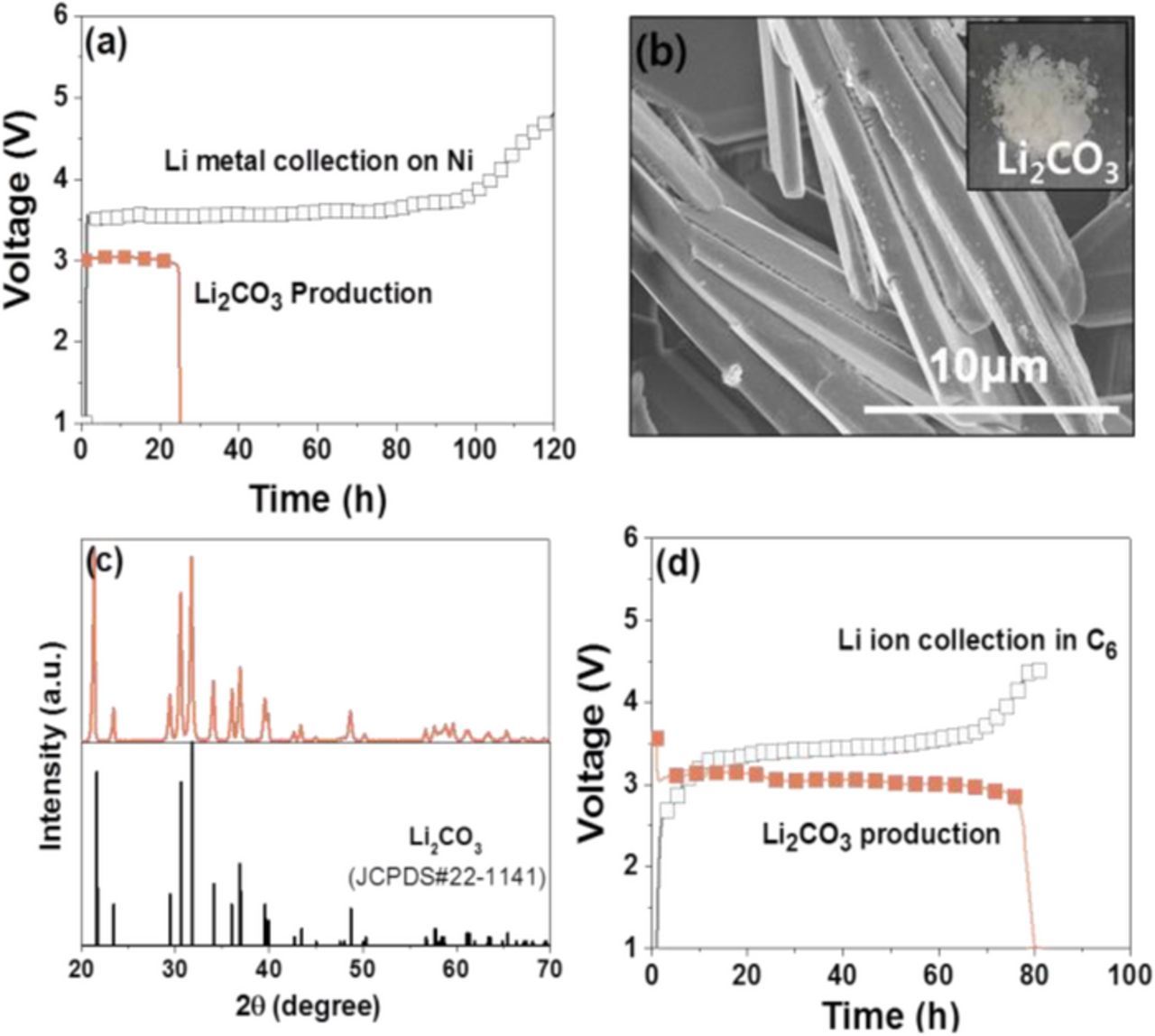
Since the Li metal is unstable and very reactive in a moisturized air atmosphere, it must be stored and carefully handled in a dry room or argon filled glove box, which limits its direct use in many applications, such as lithium grease and Li electrode materials for LIBs. For this reason, LiOH or Li2CO3 have been mostly used as the Li precursors for such applications.14 In our WTL system, air-stable Li precursor chemicals LiOH and Li2CO3 can be also obtained by simple discharge (oxidation) of the harvested Li metal in water (Fig. 1). Fig. 5a shows the charge/discharge curves of the WTL cell when using the Ni mesh current collector in the harvesting anode compartment and LiFePO4 powder in the waste cathode compartment. The empty symbol curve corresponds to the formation (harvesting) of Li metal on the Ni mesh collector. By performing the discharge process (the red solid symbol curve), Li ions pass through the LATP membrane and react with water in the recycling cathode compartment. The discharge curve plateau ∼3.0 V is thought to be due to the following chemical reaction.
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/163/7/E199/revision1/d0001.gif)
According to the above reaction, the discharge product in the recycling cathode compartments would be LiOH. The white powder of the discharge product (Fig. 5b) was collected from the recycling cathode compartment after discharging the WTL cell. However, the XRD measurement identified its phase as Li2CO3 rather than LiOH, as shown in Fig. 5c. It is thus believed that the LiOH (aq) reacts with CO2 dissolved in water, forming Li2CO3 (aq), according to the following reaction route:
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/163/7/E199/revision1/d0002.gif)
It is noted that CO2 is soluble in water and present in the form of a dissolved gas, and its solubility (1.45g/L) at room temperature is ∼200 times of that of O2 (0.0083g/L). The formation of Li2CO3 in LiOH (aq) solution is well known.23 Li2CO3 (s) powder was obtained after drying the discharged water solution in a vacuum oven at 80 °C. The surface morphology and XRD powder pattern of the Li2CO3 powder are shown in Figs. 5b and 5c, respectively. The photograph of the acquired Li2CO3 powder is shown in the inset of Fig. 5b. The particles had a pillar shape. As shown in Fig. 5c, the XRD pattern was in agreement with a typical Li2CO3 phase [JCPDS #22-1141]. The final production yield of Li2CO3 powder from the harvested Li metal was estimated to be ∼25%, based on the coulombic efficiency of the charge/discharge curves (Fig. 5a). This low yield could be due to the use of Ni mesh as the current collector, where Li metal is deposited during charging process. It is believed that small amounts of initially formed Li metal on the current collector could be easily oxidized via reactions with the liquid electrolyte and form byproducts, such as a solid-electrolyte interface (SEI) layer,24 consequently leading to the loss of the harvested Li metal. To resolve the problem and improve the Li-harvesting efficiency of the WTL system, a representative Li-intercalation electrode, graphite (C6), was employed as the Li-harvesting agent, which leads to Li ion storage instead of Li metal formation. Fig. 5d shows the charge and discharge curves of the WTL cell using a graphite (C6) electrode in the recycling anode compartment and LiFePO4 electrode in the waste cathode compartment. During the charging process, the Li ions extracted from LiFePO4 are stored in the structure of graphite through the electrochemical chemical reaction below.
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/163/7/E199/revision1/d0003.gif)
By the subsequent discharge process, the stored Li ions in the graphite anode are converted into LiOH (aq) via electrochemical reactions with water and oxygen as below.
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/163/7/E199/revision1/d0004.gif)
The formed LiOH (aq) reacts with carbon dioxide to form Li2CO3 (aq) as mentioned in reaction 2. Graphite is a well-known anode material for Li ion batteries because it can reversibly intercalate and de-intercalate Li ions within its structure through the stable SEI layers on its surface.25 As a result, the Li-harvesting efficiency of the WTL cell was significantly improved, up to ∼98%, when the Ni was replaced by the graphite anode as shown in Fig. 5d. Besides, other Li-intercalation and Li-alloy materials which have been investigated as the anodes for Li-ion batteries,26,27 can be applied as the harvesting anode for the WTL system to improve its performance.
Figure 5. (a) Charge and discharge voltage curves of the WTL cell using Ni as the current collector for Li metal formation and LiFePO4 as the Li-containing waste material. (b) SEM images, photograph (inset), and (c) XRD pattern of the discharge product of Li2CO3 powder. (d) Charge and discharge voltage curves of the WTL cell using a graphite electrode in the harvesting anode compartment and LiFePO4 as the Li-containing waste material.
The feasibility of the WTL system was also tested by using real waste Li-ion batteries. Fig. 6a shows the photograph of the selected waste Li-ion battery pack. From our chemical analysis (not shown), it is assumed that it contains a graphite anode, LiCoO2 cathode, and carbonate-based liquid electrolyte. After disassembling the waste Li-ion battery, all parts of the battery, including pouch film, current collectors, electrodes, separator, and liquid electrolytes, were subjected to sonication in distilled water to separate the electrode materials from the current collectors (Fig. 6b). Finally, a solution in which to disperse the electrode powders and Li salts from the liquid electrolyte was prepared after removing the remaining compartments of the battery (Fig. 6c). This solution was then poured into the waste cathode compartment of the WTL cell with a graphite anode and the charge process was performed. Fig. 6d shows the charge/discharge curves of the WTL cell with the water containing waste Li materials for 100 h at 0.1 mA/cm2. The charge voltage plateau (the empty symbol curve) was observed at ∼4.0 V. This indicates that the most of Li was harvested from the LiCoO2 cathode particles and inserted in the graphite anode. The discharge curve (the solid symbol) had a voltage plateau of ∼3.0 V, which could be attributed to the formation of LiOH by the electrochemical reaction with water (Reaction 4). After discharging the WTL cell, the discharge products were collected from the recycling cathode compartment and identified as Li2CO3. These results demonstrate that the WTL cell can work as an efficient and novel device for Li harvesting and recycling with commercially available Li2CO3 from various waste Li-containing materials, including waste Li-ion batteries.
Figure 6. Photographs of (a) waste Li-ion battery consisting of graphite as the anode and LiCoO2 as the cathode, (b) the disassembled battery immersed in distilled water, and (c) the waste-materials-dispersed aqueous solution. (d) Charge and discharge voltage curves for the WTL cell using a graphite electrode in the harvesting anode compartment and the waste Li-ion battery components (c) in the waste cathode compartment.
Conclusions
The WTL system was developed to recycle waste Li or Li-containing materials in a simple and environmentally friendly electrochemical process. This system operates at room temperature without using any acid solution. It was demonstrated that high-purity (∼99%) Li metal, comparable to commercial Li metal products, can be harvested from the Li-containing waste materials or stored in the Li-intercalation electrode in the harvesting anode compartment during the charging process. More than 75% of Li in the Li-containing waste materials was found to be extracted, on the basis of the Coulombic efficiency and XRD analysis. In discharging, the harvested Li metal can be transformed to commercially applicable Li2CO3 via electrochemical reactions with O2 and CO2 dissolved in water. The WTL system features straightforward, one-step electrochemical processes operable at room temperature, compared to existing complicated, energy-intensive processes for Li metal production. In addition, the system can be easily scaled up due to the simplicity of the device. Therefore, the WTL system shows great promise as a low-cost, efficient Li-recycling system that not only enables the production of highly pure Li metal and Li precursors, such as LiOH and Li2CO3, but also minimizes environmental impacts.
Acknowledgments
The work performed at Ulsan National Institute of Science and Technology was financially supported by National Research Foundation of Korea (NRF-2014R1A2A1A11052110).