Saltwater as the energy source for low-cost, safe rechargeable batteries
- Journal
- Journal of Materials Chemistry A
- Vol
- 4
- Page
- 7207-7213
- Year
- 2016
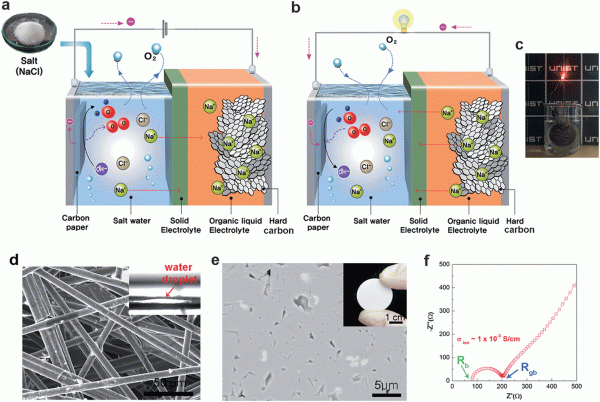
The effective use of electricity from renewable sources requires large-scale stationary electrical energy storage (EES) systems with rechargeable high-energy-density, low-cost batteries. We report a rechargeable saltwater battery using NaCl (aq.) as the energy source (catholyte). The battery is operated by evolution/reduction reactions of gases (mostly O2, with possible Cl2) in saltwater at the cathode, along with reduction/oxidation reactions of Na/Na+ at the anode. The use of saltwater and the Na-metal-free anode enables high safety and low cost, as well as control of cell voltage and energy density by changing the salt concentration. The battery with a hard carbon anode and 5 M saltwater demonstrated excellent cycling stability with a high discharge capacity of 296 mA h ghard carbon−1 and a coulombic efficiency of 98% over 50 cycles. Compared with other battery types, it offers greatly reduced energy cost and relatively low power cost when used in EES systems.