Rechargeable Seawater Battery and Its Electrochemical Mechanism
- Journal
- ChemElectroChem
- Vol
- 2
- Page
- 328-332
- Year
- 2015
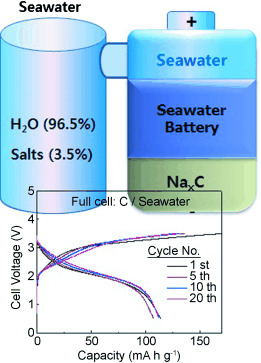
Herein, we explore the electrochemical mechanism of a novel rechargeable seawater battery system that uses seawater as the cathode material. Sodium is harvested from seawater while charging the battery, and the harvested sodium is discharged with oxygen dissolved in the seawater, functioning as oxidants to produce electricity. The seawater provides both anode (Na metal) and cathode (O2) materials for the proposed battery. Based on the discharge voltage (∼2.9 V) with participation of O2 and the charge voltage (∼4.1 V) with Cl2 evolution during the first cycle, a voltage efficiency of about 73 % is obtained. If the seawater battery is constructed using hard carbon as the anode and a Na super ion conductor as the solid electrolyte, a strong cycle performance of 84 % is observed after 40 cycles.