Chemical Stability and Degradation Mechanism of Solid Electrolytes/Aqueous Media at a Steady State for Long-Lasting Sodium Batteries
- Journal
- Chemistry of Materials
- Vol
- 33
- Page
- 126-135
- Year
- 2021
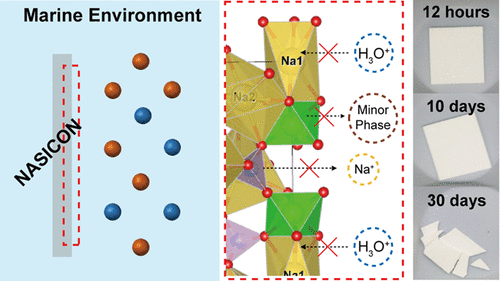
Research on the interface between solid electrolytes and electrode materials or catholyte is important to effectively and safely use their high energy densities. However, compared to interfaces with electrode materials, the interface between solid electrolytes and liquid media lacks research. Herein, the stability of NA superionic conductor (NASICON) pellets is studied in various aqueous solutions, including deionized (DI) water and a marine environment, associated with different degradation mechanisms. A representative detrimental hydronium exchange reaction between solid electrolytes and aqueous media is suppressed with increasing concentration and ion types dissolved in the solutions. Results of density functional theory calculation and electron energy loss spectroscopy reveal the different activation energies and chemical bonding states of solid electrolytes based on the aqueous solutions’ conditions. NASICON’s ionic conductivity decreases to ∼10–6 S/cm because of severe changes in aqueous solutions with insufficient dissolved ions resulting in inferior chemical stability. Furthermore, chemical stability variations at a steady state can severely affect battery performance. Seawater batteries fabricated with NASICON in immersed DI water for 1 year exhibit a large resistance region from the first cycle; this system breaks down before 200 h, unlike a cell fabricated using NASICON immersed for 1 year in a marine environment.